
Rajah Serfoji Govt. College (Autonomous)
Thanjavur – 613 005
M.Sc., Chemistry SEMESTER – III
Code: R3PCH6 Organic Chemistry - III
When sunshine in the UV-B spectrum strikes the skin, it converts a substance in the skin called 7-dehydrocholesterol into vitamin D3.2
7-dehydrocholesterol is a very close precursor to
cholesterol. If you look at our flow chart showing the synthesis of cholesterol, you will see
that it shows lanosterol being converted directly to cholesterol. This
conversion is actually believed to take more than 18 different steps and hasn't
been completely figured out, so it is usually simplified as one step.3 7-dehydrocholesterol occurs very close to the end
of this conversion, so is often referred to as "cholesterol" or
"a form of cholesterol."
Figure 2: The
Chemical Structure of Vitamin D
When atmospheric conditions are ideal and skies are
clear, 30 minutes of whole-body exposure of pale skin to sunlight without
clothing or sunscreen can result in the synthesis of between 10,000 and 20,000
IU of vitamin D. These quantities of vitamin D are large, and therefore capable
of supplying the body's full needs.2
At the same time, the body has two mechanisms to
prevent an excess of vitamin D from developing: first, further irradiation
converts excess vitamin D in the skin to a variety of inactive metabolites;
second, the pigment melanin begins to accumulate in skin tissues after the
first exposure of the season, which decreases the production of vitamin D.2
The availability of UV-B rays, however, depends on
the angle at which sunshine strikes the earth, making vitamin D synthesis
impossible for most people at most latitudes during parts of the year called
the "vitamin D winter."4
Outside the vitamin D winter, sufficient UV-B rays
for full vitamin D synthesis do not suddenly become available: the window of
time during each day in which vitamin D synthesis can occur gradually expands
as the season progresses, as does the amount of UV-B radiation available within
that window.4
Many different factors can make the availability of
UV-B widely variable during any given time of the year. Clouds alone, for
example, can eliminate up to 99 percent of UV-B radiation.5
Natural variations in the density of the ozone
layer can cause the length of the vitamin D winter to increase or decrease by
up to two months. Aerosols and buildings block UV-B radiation, while increased
altitude or reflective surfaces such as snow increase exposure to UV-B
radiation.5
In the past, researchers suggested that any place
outside of 34 degrees latitude experiences some degree of vitamin D winter,
that the vitamin D winter in Boston extended for four months from November
through February, and that the vitamin D winter in Edmonton extended for six
months from October through March.5
More recently, researchers found that so many
factors influence the availability of UV-B light that vitamin D winters under
some conditions in Boston and Edmonton could be much shorter, whereas under
other conditions, vitamin D winters can even occur at the equator.5
Since most of us live at latitudes that are covered
by a vitamin D winter for at least part of the year, and since most of us work
indoors and wear clothing and sunblock when outdoors in the summer sun, it is
necessary for most of us to consume vitamin D in food for at least part of the
year, or to supplement with vitamin D.
In order to consume vitamin D as food, we must eat
the cholesterol-rich animal foods we are so often
told to avoid.
Bioactive vitamin D or calcitriol is a steroid hormone
that has long been known for its important role in regulating body levels of
calcium and phosphorus, and in mineralization of bone. More recently, it has
become clear that receptors for vitamin D are present in a wide variety of
cells, and that this hormone has biologic effects which extend far beyond
control of mineral metabolism.
---------------------------------------------------------------------------------------------------------------------------------
The term vitamin D is, unfortunately, an imprecise term
referring to one or more members of a group of steroid molecules. Vitamin D3,
also known as cholecalciferol is generated in the skin of animals when
light energy is absorbed by a precursor molecule 7-dehydrocholesterol. Vitamin
D is thus not a true vitamin, because individuals with adequate exposure to
sunlight do not require dietary supplementation. There are also dietary sources
of vitamin D, including egg yolk, fish oil and a number of plants. The plant
form of vitamin D is called vitamin D2 or ergosterol. However,
natural diets typically do not contain adequate quantities of vitamin D, and
exposure to sunlight or consumption of foodstuffs purposefully supplemented
with vitamin D are necessary to prevent deficiencies.
Vitamin D, as either D3 or D2, does
not have significant biological activity. Rather, it must be metabolized within
the body to the hormonally-active form known as 1,25-dihydroxycholecalciferol.
This transformation occurs in two steps, as depicted in the diagram to the
right:
- Within
the liver, cholecalciferal is hydroxylated to 25-hydroxycholecalciferol
by the enzyme 25-hydroxylase.
- Within
the kidney, 25-hydroxycholecalciferol serves as a substrate
for 1-alpha-hydroxylase, yielding 1,25-dihydroxycholecalciferol,
the biologically active form.
Each of the forms of vitamin D is hydrophobic, and is
transported in blood bound to carrier proteins. The major carrier is called,
appropriately, vitamin D-binding protein. The halflife of
25-hydroxycholecalciferol is several weeks, while that of
1,25-dihydroxycholecalciferol is only a few hours.
Control of Vitamin D Synthesis
Hepatic synthesis of 25-hydroxycholecalciferol is only
loosely regulated, and blood levels of this molecule largely reflect the amount
of amount of vitamin D produced in the skin or ingested. In contrast, the
activity of 1-alpha-hydroxylase in the kidney is tightly regulated and serves
as the major control point in production of the active hormone. The major
inducer of 1-alpha-hydroxylase is parathyroid
hormone; it is also induced by low blood levels of phosphate.
Interesting species differences exist in the ability to
synthesize vitamin D through the sunlight-mediated pathway described above. The
skin of humans, horses, pigs, rats, cattle and sheep contain adequate quantities
of 7-dehydrocholesterol which can effectively be converted to cholecalciferol.
In contrast, the skin of dogs and cats constains significantly lower quantities
of 7-dehydrocholesterol than other species, and its photochemical conversion to
cholecalciferol is quite inefficient; dogs and cats thus appear to rely on
dietary intake of vitamin D more than do other animals.
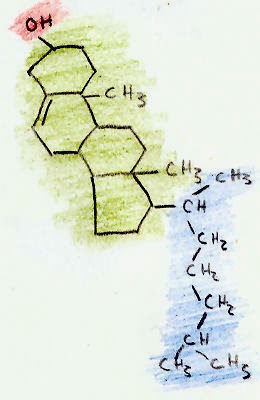
Cholesterol has a molecular formula of C27H45OH. This molecule is composed of three regions (shown in the picture
above): a hydrocarbon tail (shown in blue), a ring structure region with 4
hydrocarbon rings (shown in green), and a hydroxyl group (shown in red.).
The hydroxyl (OH) group is polar, which makes it soluble in
water. This small 2-atom structure makes cholesterol an alcohol. The
alcohol that we drink, ethanol, is a much smaller alcohol that also has a
hydroxyl group (C2H5OH).
The 4-ring region of cholesterol is the signature
of all steroid hormones (such as testosterone and estrogen). All steroids are
made from cholesterol. The rings are called "hydrocarbon" rings because each
corner of the ring is composed of a carbon atom, with two hydrogen atoms
extending off the ring.
The combination of the steroid ring structure and
the hydroxyl (alcohol) group classifies cholesterol as a "sterol."
Cholesterol is the animal sterol. Plants only make trace amounts of
cholesterol, but make other sterols in larger amounts.
The last region is the hydrocarbon tail. Like the steroid ring region, this region is
composed of carbon and hydrogen atoms. Both the ring region and tail region are
non-polar, which means they dissolve in fatty and oily substances but will not
mix with water.
Because cholesterol contains both a water-soluble
region and a fat-soluble region, it is called amphipathic.
Cholesterol, however, is not water-soluble enough
to dissolve in the blood. Along with fats and fat-soluble nutrients, therefore,
it travels in the blood through lipoproteins such as LDL and HDL.


No comments:
Post a Comment