Rajah Serfoji Govt. College (Autonomous), Thanjavur – 613 005
M.Sc., Chemistry
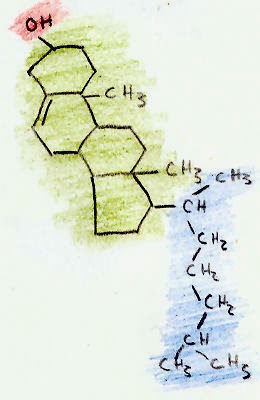
Estrone (E1, and also oestrone) is an estrogenic hormone secreted by the ovary as well as adipose tissue with the chemical name of 3-hydroxyestra-1,3,5(10)-triene-17-one and the chemical formula C18H22O2. Estrone is an odorless, solid crystalline powder, white in color with a melting point of 254.5 °C and a specific gravity of 1.23. Estrone is one of several natural estrogens, which also include estriol andestradiol. Estrone is the least abundant of the three hormones; estradiol is present almost always in the reproductive female body, and estriol is abundant primarily duringpregnancy. Estrone is known to be a carcinogen for human females as well as cause breast tenderness or pain, nausea, headache, hypertension, and leg cramps.[1][4] In men, estrone has been known to cause anorexia, nausea, vomiting, and erectile disfunction. Estrone is relevant to health and disease states because of its conversion to estrone sulfate, a long-lived derivative. Estrone sulfate acts as a reservoir that can be converted as needed to the more active estradiol. It is the predominant estrogen in postmenopausal women.
M.Sc., Chemistry
Code: R3PCH6 Organic Chemistry - III
UNIT - V SteroidsEstrone (E1, and also oestrone) is an estrogenic hormone secreted by the ovary as well as adipose tissue with the chemical name of 3-hydroxyestra-1,3,5(10)-triene-17-one and the chemical formula C18H22O2. Estrone is an odorless, solid crystalline powder, white in color with a melting point of 254.5 °C and a specific gravity of 1.23. Estrone is one of several natural estrogens, which also include estriol andestradiol. Estrone is the least abundant of the three hormones; estradiol is present almost always in the reproductive female body, and estriol is abundant primarily duringpregnancy. Estrone is known to be a carcinogen for human females as well as cause breast tenderness or pain, nausea, headache, hypertension, and leg cramps.[1][4] In men, estrone has been known to cause anorexia, nausea, vomiting, and erectile disfunction. Estrone is relevant to health and disease states because of its conversion to estrone sulfate, a long-lived derivative. Estrone sulfate acts as a reservoir that can be converted as needed to the more active estradiol. It is the predominant estrogen in postmenopausal women.
Estrone is synthesized via aromatase from androstenedione, a
derivative of progesterone. The
conversion consists of the de-methylation of C-19 and the aromaticity of the 'A' ring. This reaction is similar to the conversion of testosterone to estradiol.
Estrone is a white, solid
crystalline powder that is odorless. It has a melting point of 254.5 °C (490
°F) and has a specific gravity of 1.23. At high temperatures estrone is combustible and the products of
combusting estrone are carbon monoxide (CO) and carbon dioxide (CO2)
Estrone has been proven to
be a known carcinogen for human females. The Occupational Safety and Health Administration (OSHA)
classifies estrone as an OSHA Select carcinogen. Exposure to estrone can cause
breast tenderness or pain, cervical hyper secretion, menstrual disorders
including menorrhagia and metrorrhagia, nausea, headache, hypertension, leg
cramps, vision disturbances, and endometriosis pain in women. Mothers lactating
can also experience a decrease in the production of breast milk.
Progesterone (pregn-4-ene-3,20-dione; abbreviated as P4) is an endogenous steroid
hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species. It
belongs to a group of steroid hormones called theprogestogens,[1] and
is the major progestogen in the body. Progesterone is also a crucial metabolic intermediate in the production other endogenous steroids,
including the sex hormones and the corticosteroids, and
plays an important role in brain function as a neurosteroid.
Willard Myron Allen co-discovered progesterone with his
anatomy professor George Washington Corner at the University of Rochester
Medical School in 1933. Allen first determined its melting point, molecular
weight, and partial molecular structure. He also gave it the name Progesterone derived fromProgestational Steroidal ketone
Like other steroids, progesterone
consists of four interconnected cyclic hydrocarbons. Progesterone contains ketone and oxygenated functional groups, as
well as two methyl branches. Like all steroid hormones,
it is hydrophobic.